An international cohort of children with systemic Juvenile idiopathic arthritis (Still’s disease) validated the systemic Juvenile Arthritis Disease Activity Score 10 (sJADAS10), a disease activity measure distinguish patients with inactive disease (ID), minimal disease activity (MiDA), moderate disease activity (MoDA), and high disease activity (HDA).
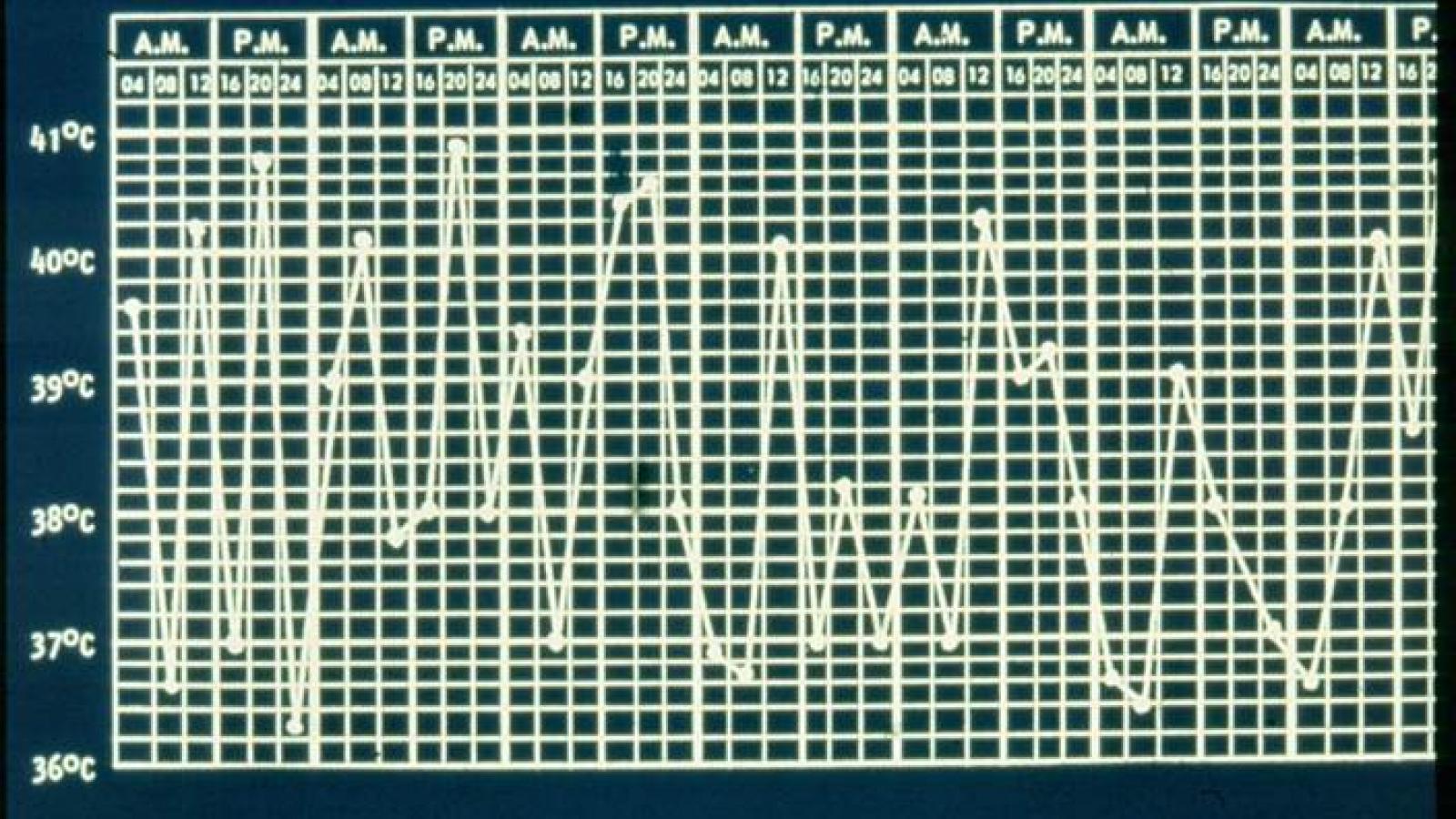
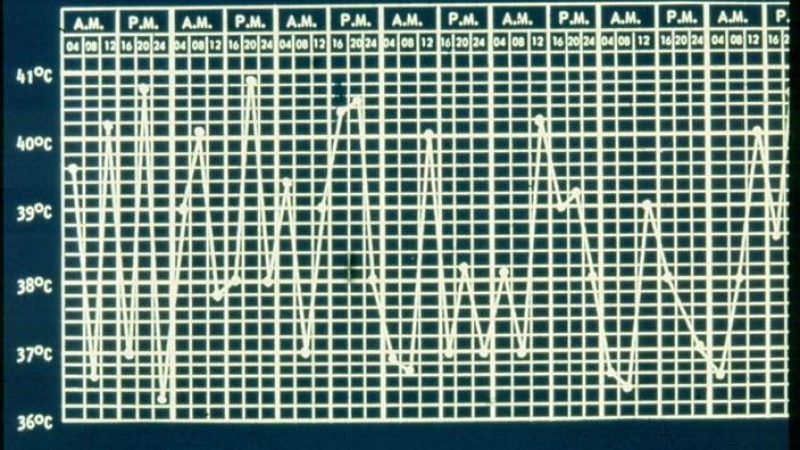
Systemic JIA accounts for nearly 15% of all JIA patients and can be difficult to diagnose or assess activity, owing to the protean manifestations of high fever, rash, arthritis and inflammatory indices. While diagnostic criteria have been established, the assessment of disease activity has not, but would be vitally important for future clinical trial conduct in Still’s disease patients.
This sJADAS10 validation exercise was developed on 400 patients enrolled at 30 pediatric rheumatology centers in 11 countries. Cutoffs were evaluated by physician rating 60% of the of the patients to defined criteria cutoffs and the remaining 40% to validate final criteria.
The sJADAS10 uses 5 key measures of disease activity (each rated on a 0-10 scale; total of 50):
- Physician global assessment (PhGA) 0-10 numerical rating scale (NRS)
- Patient Global assessment (PaGA) 0-10 NRS
- Joint counts on 10 joints
- ESR or CRP, normalized to a 0-10 scale;
- modified mSMS (ranges from 0 to 10)
- fever = 1 point if >37.5–38°C, 2 points if >38–39°C, 3 points if >39–40°C, 4 points if >40°C;
- evanescent erythematous rash = 1 point;
- generalized lymphadenopathy (enlargement of > 3 lymph node stations) = 1 point;
- hepatomegaly and/or splenomegaly = 1 point;
- serositis (pleuritis, pericarditis or peritonitis) = 1 point;
- anemia (hemoglobin <9 g/dl) = 1 point;
- platelet count >600×109/l or ferritin >500 ng/ml = 1 point.
The sJADAS10 cutoffs separating :
- Inactive disease (ID) from Minimal disease (MiDA) ≤ 2.9
- Minimal disease (MiDA) from Moderate disease (MoDA) ≤ 10
- Moderate disease (MoDA) from High activity (HDA) > 20.6
These sJADAS cutoffs were validated and are suitable for use in clinical trials and routine practice.
Related Content
-
October 1, 2018
Only inhibitors of IL-1 (canakinumab) and IL-6 (tocilizumab) are FDA approved for use…
-
January 1, 1970
Still's disease in adults (AOSD) or children (sJIA) can have dramatic symptom severity,…
-
November 26, 2019
A disease you’ve never heard of is becoming increasingly common and carries a…
-
August 8, 2019
A single-center cohort analysis shows that lung disease (LD) is increasingly seen in…
-
January 1, 1970
A multinational study of adults with Still's disease found that calculating their systemic…
-
February 20, 2019
The drug retention rate of interleukin-1 inhibitors (IL-1) used to treat systemic juvenile…